| ||||
Acids, Bases, Buffers, and the pH Scale - P2
Salts and pH Buffering
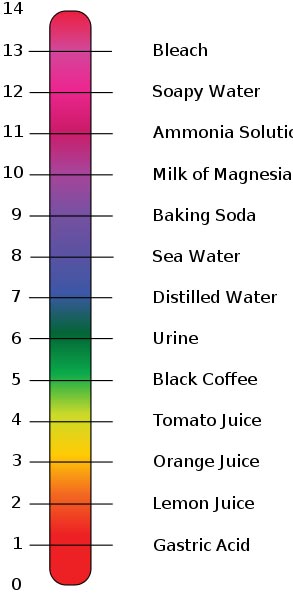
Salts are inorganic molecules can act as buffers to reduce fluctuations in pH by releasing cations and anions other than H+ and OH- in solution. An example of a salt buffer is a product like Tums the stomach antacid, which is made of the ionic compound calcium carbonate (CaCO3). Carbonate reacts with a free hydrogen ion (H+), forming bicarbonate. Then bicarbonate reacts with a free hydrogen ions to create carbonic acid (H2CO3), which then can dissociate (break down further) into water (H2O) and carbon dioxide (CO2). During this process, free hydrogen ions are locked up, preventing the pH from lowering.
SPO VIRTUAL CLASSROOMS
You have free access to a large collection of materials used in a college-level introductory Cell Biology Course. The Virtual Cell Biology Classroom provides a wide range of free educational resources including Power Point Lectures, Study Guides, Review Questions and Practice Test Questions.
Page last updated: 11/2015
PAGE 2 < Back to Page 1
Additional Resources On pH Scale
- The pH Scale animated lesson from John Kyrk.
- Acids Bases and Metals super fun interactive BBC tutorial with quiz.
- pH Scale interactive lesson and quiz from scool.co.uk
- Think of pH as Water Balance animated tutoral, Springfield Technical Community College.
VIDEO
pH
from Crash Course Chemistry