| ||||
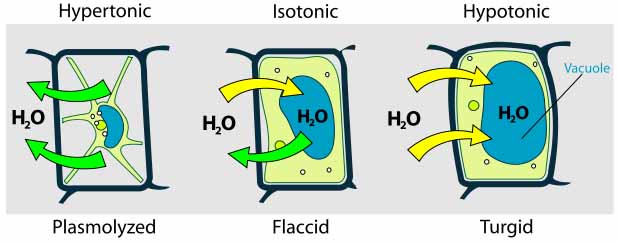
Hypertonic Environment
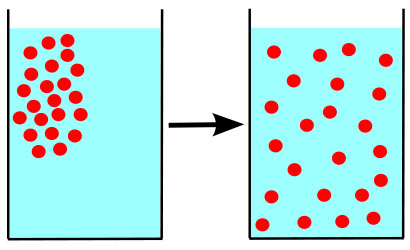
All cells are vulnerable to dehydration, as seen in plants that are not watered enough, or any cell surrounded by a hypertonic environment. When water leaves a cell, it is not as plump, although the structure of the cell wall prevents dehydrated cells from losing their shape entirely. Some cells have a waxy covering, such as plants adapted to dry environments.
Exposure to extremely hypertonic environments can also kill a plant, such as when a dog creates spots of dead lawn where it pees.
The urine is hypertonic compared to the interior of the grass cells. Water always moves towards a hypertonic environment. So in this tonicity situation, water is drawn out of the grass, killing it.
SPO VIRTUAL CLASSROOMS
More Osmosis and Diffusion Links
- Diffusion, Osmosis & Active Transport Lecture Main Page from the Virtual Cell Biology Classroom.
- How Diffusion Works animation and quiz from McGraw-Hill.
- The Cell: Passive Transport Diffusion from Wisc-Online.com.
- How Osmosis Works animation and quiz from McGraw-Hill.
- The Cell: Passive Transport Osmosis from Wisc-Online.com.
- Osmosis animation from St. Olaf College.
- Plasmolysis animation and quiz from McGraw-Hill.
Osmosis In Cells With a Cell Wall
fungi and other cells that have cell walls are subjected to osmostic pressure, just as are cells without a cell wall (such as animal cells and protozoans).
Hypotonic Environment
Plant, fungi and bacterial cells are surrounded by a strong, rigid cell wall that prevents the cell from taking in too much water and exploding.
When a cell that has a cell walls is exposed to water, the water moves into the cell, making it plump (turgid). This is why house plants look healthy and firm when they are watered sufficiently.
Diffusion, Osmosis & Tonicity - P3
PAGE 3 < Back to Page 2
Page last updated: 3/2016
Microbes in Hypertonic Environments
Although the cell walls of bacteria prevent them from taking on too much water in hypotonic surroundings, like all cells, microbes are vulnerable to losing water in hypertonic environments.
Above: A plant wilts when its cells are not full of water. Below: Areas of dead grass where dog urine (a hypertonic solution) plasmolyzes the grass cells.
Cells can either contain the same level of dissolved substances as their surrounding environment (an isotonic situation), or there can be a difference in concentration of solutes between the inside and outside of the cell. The area with a higher level of solutes is considered hypertonic. The area with a lower level of solutes is hypotonic.
Some microbes have special structures, such as a waxy covering or gooey slime layer, to protect them from drying out in high solute surroundings.
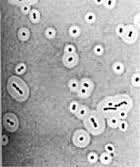
The thick halo of slime surrounding these
Streptococcus
protect them from drying out in hypertonic environments.
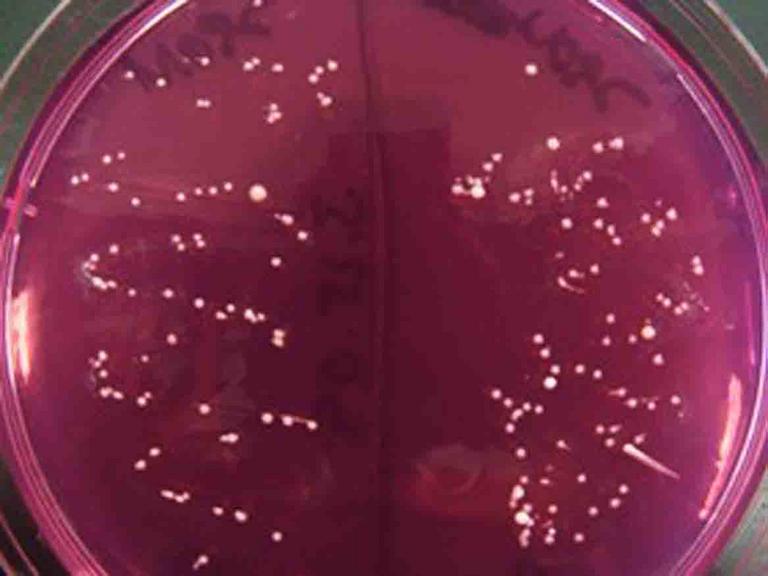
Mannitol Salt Agar is a specialized bacterial growth medium that has a high concentration of salt. Only halophilic (salt-loving) bacteria can grow on this agar, such as the tiny, punctiform Staphylococcus colonies pictured above. In the real world, Staph grows well on our salty skin and mucous membranes because of the slime layer that protects these bacteria from losing water.
End of article ...
 | ||||
Science Prof Online
has several
Virtual Classrooms
including:
(15 week)
(15 week)
(8 week)
(8 week)
(15 week)
 | ||||||
See all of related teaching materials on the